


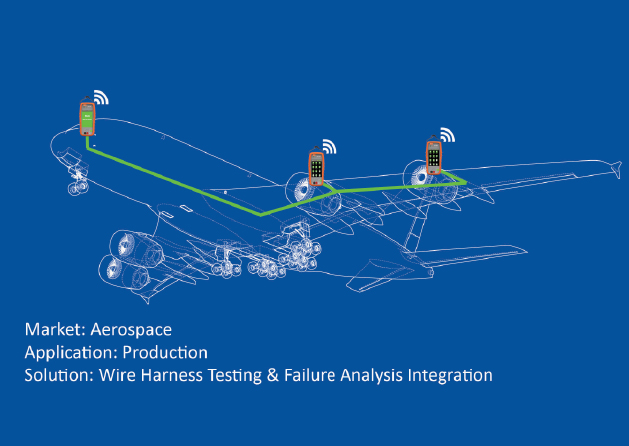
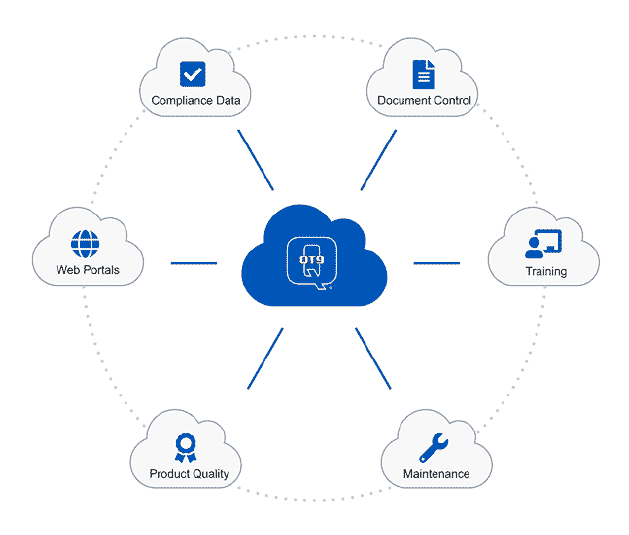
Central Repository: Document control software serves as a central location for all of your documentation, making it easier to find and deliver documents.
Audit Trail: Automated document control automatically creates an audit trail, allowing you to track the history of changes made to documents. This is especially helpful during the auditing process to demonstrate document control and quality control to regulators.
Risk Management: Document control software provides a clear record of specifications, requirements and procedures, allowing you to proactively address potential issues and contributing to better control over business outcomes.
Training and Onboarding: Having standardized and controlled documentation ensures that new employees receive accurate and consistent information, helping them integrate into their positions and contribute effectively.
Access Control: Security measures can be easily established based on named criteria, such as user, location and document lifecycle. Only verified users with authenticated permission can be given access and the documents are secure, encrypted and protected within the application.
Built-in Efficiencies: Document control software offers efficiencies such as automatically archiving obsolete documents; email alerts and reminders for changes, reviews and approvals; links to training; electronic approvals; and access to real-time data for better insight into your document control efforts.
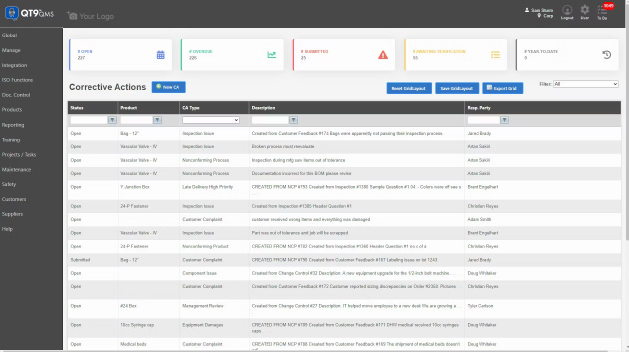
Audit management software facilitates every stage of the audit process, from planning and scheduling to generating final reports, and improving the efficiency and accuracy of the audit process. It automates the process of preparing and executing audits by helping companies analyze data, assess risks, track issues, report results and centralize documentation. Regulatory compliance is streamlined and simplified with centralized workflows and full traceability.
